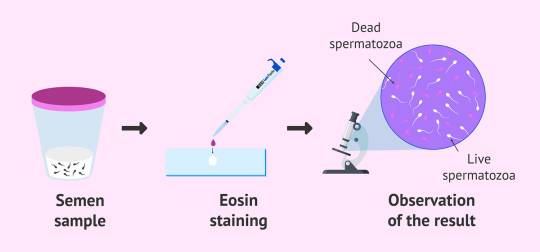
warning: this is not how to include the shop layout in a page, please read docs
Preview
Image
name
description
category
price
How to install
You can now add layouts you create in the Shop Admin into any post or page. To make it simple a Shortcode Generator is included. You can access it via this button.
The first button is the shortcode generator launcher and the second one ( the one with a plus ) ads a single audio player to the page from the media gallery.